eureKING Project
Biomanufacturing is the Next European Frontier
The creation of a new European biopharma CDMO champion will target a booming yet highly fragmented industry. Led by a seasoned management team, eureKING will act on well identified targets allowing a timely and successful execution of the investment strategy.
A European opportunity
- Untapped market opportunity
- High growth market from a significant shortage of specialised manufacturing and development capacity
- Small to mid-sized biotechs are not well addressed by large CDMOs
- Limited attention from Private Equity in Europe
- Need for local facilities and versatile structures for another listed player
Three high-growth segments
- Biologics
- Cell & gene therapy
- Live biotherapeutics
A unique investment proposition
- The biopharmaceutical market is booming and is shaping the pharma sector’s future
- Biopharma CDMOs as opportunity to invest into the manufacturing stage of this fast-growing sector without taking the binary risks associated with individual clinical trials
- Strong pipeline and organic / external growth potential
- Proven execution track-record
- Attractive and healthy margins
- Value creation potential brought by Founders’ expertise
- Substantial consolidation potential
eureKING Will Target Three High-Growth Segments
BIOLOGICS

’20-’25
- Protein-based drugs and vaccines produced by living cells.
- Relatively mature segment with wel-established CDMO players.
- Very dynamic witdh significant needs for smallscale bioreactors and high fragmentation.
Biologic FDA approvals over time and pipeline
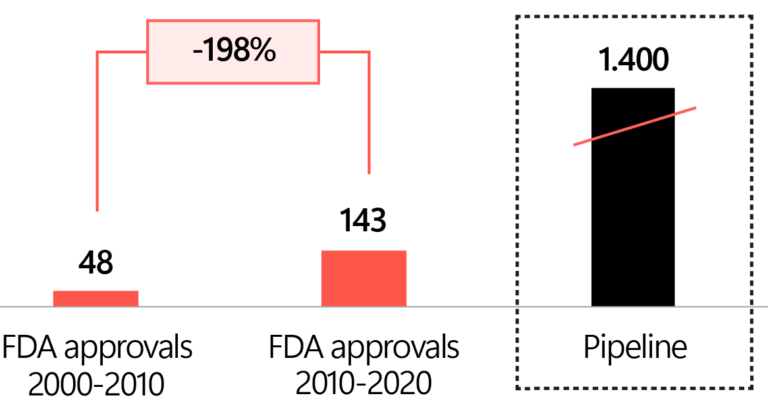
Source: FDA. BioProcess Internationnal
BIOLOGICS

’20-’25
- Protein-based drugs and vaccines produced by living cells.
- Relatively mature segment with wel-established CDMO players.
- Very dynamic with significant needs for small-scale bioreactors and high fragmentation.
Biologic FDA approvals over time and pipeline

Source: FDA. BioProcess Internationnal
CELL & GENE THERAPY

’21-’23
- Gene therapy is an approach to treat diseases by modifying a gene sequence.
- Cell therapy involves the transplantation of material into a patient.
- Long-term trend amplified by the fast-track approval of first mRNA products.
We anticipate that by 2020 we will be receiving more than 200 INDS [in gene and cell therapy] per year, building upon our total of more 800 active cell-based or directly administered gene therapy INDS currently on file with the FDA. And by 2025, we predict that the FDA will be approving 10 to 20 cell and gene therapy products a year.
CELL & GENE THERAPY

’21-’23
- Gene therapy is an approach to treat diseases by modifying a gene sequence.
- Cell therapy involves the transplantation of material into a patient.
- Long-term trend amplified by the fast-track approval of first mRNA products.
We anticipate that by 2020 we will be receiving more than 200 INDS [in gene and cell therapy] per year, building upon our total of more 800 active cell-based or directly administered gene therapy INDS currently on file with the FDA. And by 2025, we predict that the FDA will be approving 10 to 20 cell and gene therapy products a year.
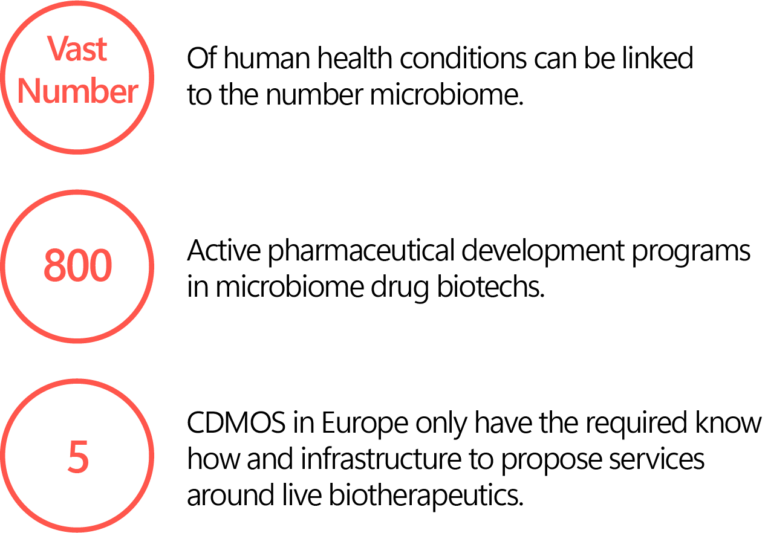
LIVE BIOTHERAPEUTICS

’25-’28
- Linked to the microbiome therapeutics segment which is targeting all diseases linked to the dysfunction and imbalance of the microbiome.
- Europe is at the R&D forefront.
- Still very limited number of expert CDMO providers today with room for huge capacity expansion
LIVE BIOTHERAPEUTICS

’25-’28
- Linked to the microbiome therapeutics segment which is targeting all diseases linked to the dysfunction and imbalance of the microbiome.
- Europe is at the R&D forefront.
- Still very limited number of expert CDMO providers today with room for huge capacity expansion

